Difference between revisions of "Aquatic Macroinvertebrates"
Cellsworth (Talk | contribs) |
Cellsworth (Talk | contribs) |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 17: | Line 17: | ||
</table> | </table> | ||
| − | [[File: | + | [[File:IMG_2066.JPG|thumb|center|500px|]] |
<!-- | <!-- | ||
| Line 45: | Line 45: | ||
==Midges (Chironomidae)== | ==Midges (Chironomidae)== | ||
| − | Midges are small, delicate flies, resembling mosquitoes but do not bite. Larvae are usually found in sediments, and can occur in everything ranging from highly polluted conditions to relatively clean water. [https://bugguide.net/node/view/3163] [http://www.chironomidae.net/index.html] The chironomid fauna of the Colorado River in Grand Canyon is depauperate in comparison with other western rivers (e.g., Sublette | + | Midges are small, delicate flies, resembling mosquitoes but do not bite. Larvae are usually found in sediments, and can occur in everything ranging from highly polluted conditions to relatively clean water. [https://bugguide.net/node/view/3163] [http://www.chironomidae.net/index.html] |
| − | and Sublette 1979, Wolz and Shiozawa 1995, Spindler 1996). Our collections include 38 species in 23 genera and 4 subfamilies. The fauna is dominated by the Orthoeladiinae (23 species), with 5 abundant species: Cricotopus annulator > Cricotopus globistylus > Eukiefferiella claripennis > Orthocladius rivicola > Tvetenia vitracies. The fauna includes 12 Chironominae species, with Chironomtts spp. regularly found in low densities in pool and backwater habitats floored with fine sand or silt, and with Procladius bellus, Paracladius conversus, Chironornus decorus, C. sp. 1, and C. sp. 2 collected only in the ULM segment. [https://scholarsarchive.byu.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=3242&context=gbn] | + | |
| + | The chironomid fauna of the Colorado River in Grand Canyon is depauperate in comparison with other western rivers (e.g., Sublette and Sublette 1979, Wolz and Shiozawa 1995, Spindler 1996). Our collections include 38 species in 23 genera and 4 subfamilies. The fauna is dominated by the Orthoeladiinae (23 species), with 5 abundant species: Cricotopus annulator > Cricotopus globistylus > Eukiefferiella claripennis > Orthocladius rivicola > Tvetenia vitracies. The fauna includes 12 Chironominae species, with Chironomtts spp. regularly found in low densities in pool and backwater habitats floored with fine sand or silt, and with Procladius bellus, Paracladius conversus, Chironornus decorus, C. sp. 1, and C. sp. 2 collected only in the ULM segment. [https://scholarsarchive.byu.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=3242&context=gbn] | ||
[[File:Midge.jpg|thumb|right|400px|Midge (larvae)]] [[File:Insect_leaf.png|thumb|right|400px|Midge (adult)]] | [[File:Midge.jpg|thumb|right|400px|Midge (larvae)]] [[File:Insect_leaf.png|thumb|right|400px|Midge (adult)]] | ||
| Line 52: | Line 53: | ||
====TANYPODINAE==== | ====TANYPODINAE==== | ||
| − | * | + | *Procladius bellus |
====DIAMESlNAE==== | ====DIAMESlNAE==== | ||
| − | * | + | *Diamesia heteropus |
| − | ==== | + | ====ORTHOCLADIINAE==== |
| − | * | + | *Cardiocladius platypus |
| − | * | + | *Cricotopus annulator |
| − | * | + | *Crirotopus blinni |
| − | * | + | *Crirotopus globistylus |
| − | * | + | *Crirotopus herrmanni |
| − | * | + | *Crirotopus infuscatus |
| − | * | + | *Cricotopus trifacsia |
*Undet. Cricotopus sp. | *Undet. Cricotopus sp. | ||
| − | * | + | *Eudactylocladius dubitatus |
| − | * | + | *Eukiefferiella claripennis |
| − | * | + | *Eukiefferiella coerulescens |
| − | * | + | *Eukiefferiella ilkeyensis |
| − | *Undet. | + | *Undet. Eukiefferiella sp. |
| − | *Undet. | + | *Undet. Limnophyes sp. |
| − | * | + | *Metriocnemus stevensi |
| − | * | + | *Orthocladius frigidus |
*Orthocladius lutipes | *Orthocladius lutipes | ||
| − | * | + | *Orlhocladius mallochi |
| − | * | + | *Orthocladius rivicola |
| − | *Undet. | + | *Undet. Orthocladius sp. |
| − | * | + | *Paracladius conversus |
| − | * | + | *Parakiefferiella subaterrima |
| − | * | + | *Parametriocnemus lundbeckii |
| − | * | + | *Paraphaenocladius exagitans |
| − | * | + | *Pseudosmittia nanseni |
*Undet. Pseudosmittia sp. | *Undet. Pseudosmittia sp. | ||
| − | *Tvetenia | + | *Tvetenia vitracies |
| − | + | ||
| − | ==== | + | ====CHIRONOMlNAE <br>==== |
| − | + | Chironomini | |
*Apedilum subcinctum | *Apedilum subcinctum | ||
| − | * | + | *Chironomus decorus |
| − | * | + | *Chironomus utahensis |
| − | * | + | *Chironomus sp. 1 |
*Chironomus sp. 2 | *Chironomus sp. 2 | ||
| − | * | + | *Cyphonoe1la gibbera |
| − | * | + | *Phaenospectra profusa |
| − | * | + | *Polypedilum apicatuim |
| − | * | + | *Polypedilum obelos |
| − | *Undet. | + | *Undet. Polypedilum sp. |
Tanytarsini | Tanytarsini | ||
| − | * | + | *Cladotanytarsus marki |
| − | * | + | *Rheotanytarsus hamatus |
| − | *Undet. | + | *Undet. Micropsectra sp. |
| − | + | ||
==Blackflies (Simuliidae)== | ==Blackflies (Simuliidae)== | ||
| + | [[File:Blackfly.jpg|thumb|right|400px|Blackfly (larvae)]] | ||
Blackflies are sometimes called a buffalo gnat, turkey gnat, or white socks. Most black flies gain nourishment by feeding on the blood of mammals, including humans, although the males feed mainly on nectar. They are usually small, black or gray, with short legs, and antennae. They are a common nuisance for humans, and many U.S. states have programs to suppress the black fly population. Eggs are laid in running water, and the larvae attach themselves to rocks. Breeding success is highly sensitive to water pollution.[2] The larvae use tiny hooks at the ends of their abdomens to hold on to the substrate, using silk holdfasts and threads to move or hold their place. They have foldable fans surrounding their mouths. The fans expand when feeding, catching passing debris (small organic particles, algae, and bacteria). The larva scrapes the fan's catch into its mouth every few seconds. Black flies depend on lotic habitats to bring food to them. They will pupate under water and then emerge in a bubble of air as flying adults. They are often preyed upon by trout during emergence. [https://en.wikipedia.org/wiki/Black_fly] | Blackflies are sometimes called a buffalo gnat, turkey gnat, or white socks. Most black flies gain nourishment by feeding on the blood of mammals, including humans, although the males feed mainly on nectar. They are usually small, black or gray, with short legs, and antennae. They are a common nuisance for humans, and many U.S. states have programs to suppress the black fly population. Eggs are laid in running water, and the larvae attach themselves to rocks. Breeding success is highly sensitive to water pollution.[2] The larvae use tiny hooks at the ends of their abdomens to hold on to the substrate, using silk holdfasts and threads to move or hold their place. They have foldable fans surrounding their mouths. The fans expand when feeding, catching passing debris (small organic particles, algae, and bacteria). The larva scrapes the fan's catch into its mouth every few seconds. Black flies depend on lotic habitats to bring food to them. They will pupate under water and then emerge in a bubble of air as flying adults. They are often preyed upon by trout during emergence. [https://en.wikipedia.org/wiki/Black_fly] | ||
| − | |||
| − | |||
==Mayflies (Ephemeroptera)== | ==Mayflies (Ephemeroptera)== | ||
| Line 121: | Line 119: | ||
The imago (the final adult stage) has shiny, hairless wings. The longer legs and tails allow for more rapid flight. The corrugation of the wings protects them by making them more flexible and therefore less vulnerable to wind damage. The imago mates and dies within a few hours to a day. (Harker, 1989) This short adult life is what gives the order its name from the Greek ephemeros meaning "lasting but a day." [https://ucmp.berkeley.edu/arthropoda/uniramia/ephemeroptera.html] | The imago (the final adult stage) has shiny, hairless wings. The longer legs and tails allow for more rapid flight. The corrugation of the wings protects them by making them more flexible and therefore less vulnerable to wind damage. The imago mates and dies within a few hours to a day. (Harker, 1989) This short adult life is what gives the order its name from the Greek ephemeros meaning "lasting but a day." [https://ucmp.berkeley.edu/arthropoda/uniramia/ephemeroptera.html] | ||
| − | ===Mayflies detected or likely to occur in Grand Canyon === | + | ===Mayflies detected or likely to occur in Grand Canyon [http://gcdamp.com/images_gcdamp_com/f/fd/MNA_Year1_Final_Report_Submitted_to_TWG_180428.pdf]=== |
*Baetidae Acentrella insignificans (McDunnough 1926) | *Baetidae Acentrella insignificans (McDunnough 1926) | ||
*Baetidae Baetis adonis (Traver 1935) | *Baetidae Baetis adonis (Traver 1935) | ||
| Line 140: | Line 138: | ||
*Leptophlebiidae Thraulodes speciosus (Traver 1934) | *Leptophlebiidae Thraulodes speciosus (Traver 1934) | ||
*Leptophlebiidae Traverella albertana (McDunnough 1931) | *Leptophlebiidae Traverella albertana (McDunnough 1931) | ||
| − | |||
==Stoneflies (Plecoptera)== | ==Stoneflies (Plecoptera)== | ||
| Line 146: | Line 143: | ||
The Plecoptera are an order of insects, commonly known as stoneflies. All species of Plecoptera are intolerant of water pollution, and their presence in a stream or still water is usually an indicator of good or excellent water quality.[https://en.wikipedia.org/wiki/Plecoptera] | The Plecoptera are an order of insects, commonly known as stoneflies. All species of Plecoptera are intolerant of water pollution, and their presence in a stream or still water is usually an indicator of good or excellent water quality.[https://en.wikipedia.org/wiki/Plecoptera] | ||
| − | ===Stoneflies detected or likely to occur in Grand Canyon === | + | ===Stoneflies detected or likely to occur in Grand Canyon [http://gcdamp.com/images_gcdamp_com/f/fd/MNA_Year1_Final_Report_Submitted_to_TWG_180428.pdf]=== |
*Chloroperlidae Suwallia pallidula (Banks 1904) | *Chloroperlidae Suwallia pallidula (Banks 1904) | ||
*Chloroperlidae Sweltsa lamba (Needham and Claassen 1925) | *Chloroperlidae Sweltsa lamba (Needham and Claassen 1925) | ||
| Line 154: | Line 151: | ||
*Perlodidae Isoperla quinquepunctata (Banks 1902) | *Perlodidae Isoperla quinquepunctata (Banks 1902) | ||
*Taeniopterygidae Taeniopteryx parvula (Banks 1918) | *Taeniopterygidae Taeniopteryx parvula (Banks 1918) | ||
| − | |||
==Caddisflies== | ==Caddisflies== | ||
Caddisflies resemble moths, but wings are hairy instead of scaly. Forewings are usually dark, sturdy, sometimes with striking color patterns, held tightly together roof-like over the abdomen when at rest. Hindwings often clear, relatively delicate, and hidden under forewings when at rest. Antennae usually very long, threadlike, with many segments. The aquatic larvae have three pairs of legs and a soft, elongate, segmented abdomen usually hidden inside a case. Most species live in a mobile case constructed from plant material, algae, grains of sand, pieces of snail shells, or entirely of silk. The case is held together with strands of silk secreted by the larva. In some species the case is attached to a rock, log, or other underwater surface; a few species have no case and are free-living. The case's particular shape and construction material is distinctive of the family and/or genus, and can be used in identification. For example: Helicopyschidae larvae use sand grains to build spiral cases that resemble small snail shells. [https://bugguide.net/node/view/5233] | Caddisflies resemble moths, but wings are hairy instead of scaly. Forewings are usually dark, sturdy, sometimes with striking color patterns, held tightly together roof-like over the abdomen when at rest. Hindwings often clear, relatively delicate, and hidden under forewings when at rest. Antennae usually very long, threadlike, with many segments. The aquatic larvae have three pairs of legs and a soft, elongate, segmented abdomen usually hidden inside a case. Most species live in a mobile case constructed from plant material, algae, grains of sand, pieces of snail shells, or entirely of silk. The case is held together with strands of silk secreted by the larva. In some species the case is attached to a rock, log, or other underwater surface; a few species have no case and are free-living. The case's particular shape and construction material is distinctive of the family and/or genus, and can be used in identification. For example: Helicopyschidae larvae use sand grains to build spiral cases that resemble small snail shells. [https://bugguide.net/node/view/5233] | ||
| − | ===Caddisflies detected or likely to occur in Grand Canyon | + | ===Caddisflies detected or likely to occur in Grand Canyon [http://gcdamp.com/images_gcdamp_com/f/fd/MNA_Year1_Final_Report_Submitted_to_TWG_180428.pdf] === |
*Brachycentridae Brachycentrus occidentalis (Banks 1911) | *Brachycentridae Brachycentrus occidentalis (Banks 1911) | ||
*Brachycentridae Micrasema bactro (Ross 1938) | *Brachycentridae Micrasema bactro (Ross 1938) | ||
| Line 204: | Line 200: | ||
*Uenoidae Neothremma alicia (Dodds and Hisaw 1925) | *Uenoidae Neothremma alicia (Dodds and Hisaw 1925) | ||
*Uenoidae Oligophlebodes sierra (Ross 1944) | *Uenoidae Oligophlebodes sierra (Ross 1944) | ||
| − | |||
==Freshwater amphipods, scuds== | ==Freshwater amphipods, scuds== | ||
===Gammarus lacustris=== | ===Gammarus lacustris=== | ||
| + | [[File:GammarusLacustris.JPG|thumb|right|500px|Gammarus lacustris: an introduced non-native freshwater shrimp (amphipod)]] | ||
G. lacustris is semi-transparent and lacks a webbed tail. It may be colorless, brown, reddish or bluish in color, depending on the local environment. It has seven abdominal segments, a fused cephalothorax, and two pairs of antennae. Unlike other crustaceans, amphipods lack carapaces and have laterally compressed bodies. The female carries eggs in a brood pouch on its ventral side. G. lacustris in higher elevations were more likely to have fewer but larger eggs than those living at lower elevations. G. lacustris undergoes several molts and juveniles resemble the adult. G. lacustris plays an important role in many of the freshwater ecosystems that it inhabits. It is a detritivore and may also consume algae, mainly diatoms. It is considered an indicator species for the overall health and stability of the ecosystem. As a small aquatic invertebrate G. lacustris is an important food source for many organisms. [https://en.wikipedia.org/wiki/Gammarus_lacustris] | G. lacustris is semi-transparent and lacks a webbed tail. It may be colorless, brown, reddish or bluish in color, depending on the local environment. It has seven abdominal segments, a fused cephalothorax, and two pairs of antennae. Unlike other crustaceans, amphipods lack carapaces and have laterally compressed bodies. The female carries eggs in a brood pouch on its ventral side. G. lacustris in higher elevations were more likely to have fewer but larger eggs than those living at lower elevations. G. lacustris undergoes several molts and juveniles resemble the adult. G. lacustris plays an important role in many of the freshwater ecosystems that it inhabits. It is a detritivore and may also consume algae, mainly diatoms. It is considered an indicator species for the overall health and stability of the ecosystem. As a small aquatic invertebrate G. lacustris is an important food source for many organisms. [https://en.wikipedia.org/wiki/Gammarus_lacustris] | ||
| − | |||
| − | |||
==Clams and Mussels== | ==Clams and Mussels== | ||
| − | === | + | [[Image:Quagga Pic (1).JPG|thumb|right|400px|Quagga mussel]] |
| − | *Pisidium variable | + | ===Fingernail clams (Sphaeriidae)=== |
| − | * | + | Fingernail clams are small "mostly about the size of a finger or thumbnail" bottom-dwelling, filter-feeders found in ponds, lakes and streams. They are native and can be quite abundant, providing food for a variety of animals and producing large accumulations of empty shells. These shells can be quite fragile compared to introduced Asian clams of the family Corbiculidae. [http://fieldguide.mt.gov/speciesDetail.aspx?elcode=IMBIV51250] |
| − | ===Quagga mussel=== | + | *Triangular peaclam (Pisidium variable) [http://fieldguide.mt.gov/speciesDetail.aspx?elcode=IMBIV51230] |
| − | + | *Walker peaclam (Pisidium walkeri) [http://fieldguide.mt.gov/speciesDetail.aspx?elcode=IMBIV51250] | |
| + | ===Quagga mussel (Dreissena rostriformis bugensis)=== | ||
| + | A highly invasive mussel prone to causing biofowling of water-related infrastructure. | ||
| + | *[http://gcdamp.com/index.php?title=Quagga Quagga wiki page] | ||
| + | *[https://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=95 USGS fact sheet] | ||
==Snails== | ==Snails== | ||
===Physella=== | ===Physella=== | ||
| + | Physella is a genus of small, left-handed, air-breathing freshwater snails. | ||
===Fossaria obrussa=== | ===Fossaria obrussa=== | ||
| + | Fossaria obrussa is a species of air-breathing freshwater pond snails. | ||
===New Zealand mudsnails (Potamopyrgus antipodarum)=== | ===New Zealand mudsnails (Potamopyrgus antipodarum)=== | ||
| − | + | The New Zealand mudsnail is a tiny aquatic snail that inhabits lakes, | |
| + | rivers, streams, reservoirs and estuaries. In addition to mud, the snail can also be found lurking | ||
| + | on rock or gravel surfaces, aquatic vegetation, or woody debris. New Zealand mudsnail are | ||
| + | highly adaptable to diverse climates and can tolerate a broad range of aquatic conditions such | ||
| + | as temperature, salinity, turbidity, water velocity, and stream productivity. In the United | ||
| + | States, New Zealand mudsnail populations are comprised almost entirely of self‐cloning | ||
| + | parthenogenetic females. The brood size of an individual female | ||
| + | ranges from 20‐120 embryos, each of which may mature to produce an average of 230 | ||
| + | offspring per year. A single female mudsnail can result in a colony of 40 million snails in one year. Some fish and birds feed on New Zealand mudsnail, but | ||
| + | the rigid operculum and thick shell wall enable many snail to pass | ||
| + | through the digestive system of predators unharmed. [https://www.fws.gov/columbiariver/ANS/factsheets/mudsnail.pdf] New Zealand mudsnails were introduced below Glen Canyon Dam around 1995. | ||
==Worms== | ==Worms== | ||
===Lumbricidae=== | ===Lumbricidae=== | ||
| + | [[Image:Earthwarm.PNG|thumb|right|300px|Earthworm (Lumbricidae)]] | ||
| + | The Lumbricidae are a family of earthworms which includes most of the well-known earthworm species. | ||
===Lumbriculidae=== | ===Lumbriculidae=== | ||
| + | [[Image:Lumbriculidae.PNG|thumb|right|400px|Lumbriculidae]] | ||
| + | The Lumbriculidae are a family of microdrile oligochaetes common in freshwater environments, including streams, lakes, marshes, wells and groundwater. They should not be confused with the earthworm family Lumbricidae. Many species and genera are highly endemic. | ||
===Naididae=== | ===Naididae=== | ||
| − | + | [[Image:Naidiae.PNG|thumb|right|400px|Naidiae tubificid worms]] | |
| − | + | The Naididae (formerly known as Tubificidae) are a family of clitellate oligochaete worms like the sludge worm, Tubifex tubifex. They are key components of the benthic communities of many freshwater and marine ecosystems. In freshwater aquaria they may be referred to as detritus worms. | |
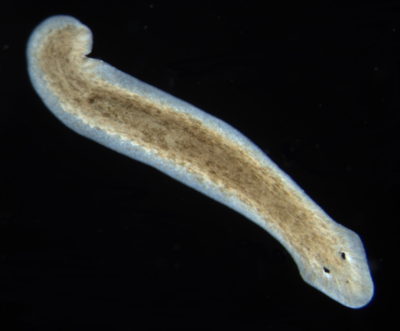
==Planaria== | ==Planaria== | ||
===Dugesia=== | ===Dugesia=== | ||
| + | [[Image:Dugesia.PNG|thumb|left|400px|Flatworm (Planaria, Dugesia) ]] | ||
| + | Dugesia (pronounced, d(y)üˈjēzh(ē)ə) is a genus of dugesiid triclads that contains some common representatives of the class Turbellaria. These common flatworms are found in freshwater habitats of Africa, Europe, Middle East, Asia, and Australia. | ||
|} | |} | ||
| Line 256: | Line 272: | ||
|style="color:#000;"| | |style="color:#000;"| | ||
| + | '''2018''' | ||
| + | *[[Media:MNA Year1 Final Report Submitted to TWG 180428.pdf| COLORADO RIVER BENTHIC FOODBASE STUDIES IN GLEN AND GRAND CANYONS: YEAR 1 FINAL REPORT Appendix 1A: Ephemeroptera, Plecoptera, and Trichoptera occurring in Grand Canyon (GC) or in the Grand Canyon ecoregion (GCE) detected (D), likely (L), or possibly (P) occurring, their EPA median tolerance score and functional feeding group (FFG; Merritt et al. 2008), elevation range (m), and range of flight dates.]] | ||
| + | |||
| + | '''1998''' | ||
*[https://scholarsarchive.byu.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=3242&context=gbn Stevens, Lawrence E.; Sublette, James E.; and Shannon, Joseph P. (1998) "Chironomidae (Diptera) of the Colorado River, Grand Canyon, Arizona, USA, II: factors influencing distribution," Great Basin Naturalist: Vol. 58 : No. 2 , Article 2. Available at: https://scholarsarchive.byu.edu/gbn/vol58/iss2/2] | *[https://scholarsarchive.byu.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=3242&context=gbn Stevens, Lawrence E.; Sublette, James E.; and Shannon, Joseph P. (1998) "Chironomidae (Diptera) of the Colorado River, Grand Canyon, Arizona, USA, II: factors influencing distribution," Great Basin Naturalist: Vol. 58 : No. 2 , Article 2. Available at: https://scholarsarchive.byu.edu/gbn/vol58/iss2/2] | ||
| + | |||
| + | '''1991''' | ||
| + | *Blinn, D. W. and G. A. Cole. 1991. Algal and invertebrate biota in the Colorado River: comparison of pre- and post-dam conditions. In: National Research Council, editor, Colorado River ecology and dam management. National Academy Press, Washington, D.C. Pp. 102–123. | ||
|- | |- | ||
| Line 263: | Line 286: | ||
|style="color:#000;"| | |style="color:#000;"| | ||
| + | ===EPT in Cataract Canyon=== | ||
| + | [http://gcdamp.com/images_gcdamp_com/4/4c/2003_Haden_benthic_structure_CR.pdf Haden et al. (2003)] found EPT in the mainstem Green and Colorado Rivers down through Cataract Canyon. Standing mass of invertebrates at each site is a function of food availability at each site. Food resource limitation has been shown to have negative effects on the standing mass of invertebrates in a detrital-based ecosystem (Wallace et al., 1999). Occurrence of EPT was much higher on cobble substrates than sandy substrates. Found that cobble substrate was relatively rare and the lack of stable substrate might be a factor limiting the overall standing mass of invertebrates. Other studies of rivers with predominantly soft sediments suggested that wood substrates were important invertebrate habitat (Benke et al., 1984; Haden et al., 1999). [http://gcdamp.com/images_gcdamp_com/4/4c/2003_Haden_benthic_structure_CR.pdf] | ||
| + | |||
| + | ===Translocation of aquatic macroinvertebrates to the Glen Canyon tailwater=== | ||
| + | Notably, several species of cold-tolerant nonnative invertebrates were intentionally introduced into the Colorado River after Glen Canyon Dam was closed in 1963. Altogether 10,000 immature mayflies were secured from a commercial source in Minnesota and released at three sites in the Lees Ferry reach. Also, 10,000 snails, 5,000 leeches, and thousands of insects representing at least 10 families were transported from the San Juan River in New Mexico to the river near Lees Ferry. In addition, 50,000 “scuds” (Gammarus lacustris) were introduced into Bright Angel Creek in 1932 and at Lees Ferry and below the dam in 1968, in addition to 2,000 crayfish taken from the LCR near Springerville, AZ. Gammarus lacustris has thrived in the cold, clear reaches below the dam, but the fate of the other introduced species is unknown. [https://www.nap.edu/read/1832/chapter/8#114 (Blinn and Cole 1991)] | ||
|} | |} | ||
Latest revision as of 15:24, 19 September 2019
|
|
Aquatic Macroinvertebrates below Glen Canyon DamThe most abundant aquatic macroinvertebrates within the Glen Canyon reach include Gammarus lacustris (an introduced non-native amphipod), midges (order Diptera, family Chironomidae), snails (Physella sp. and Fossaria obrussa), segmented worms (especially Lumbricidae and Lumbriculidae) and other aquatic worms (Naididae and Tubificidae), fingernail clams in the family Sphaeriidae (Pisidium variable and P. walkeri), and the planarian Dugesia spp. (Blinn et al. 1992; Stevens, Shannon et al. 1997). Prior to 1995, snails were infrequently observed, but have since increased in abundance due to invasion by the non-native New Zealand mudsnail (Potamopyrgus antipodarum) (Valdez and Speas 2007; Cross et al. 2010). The mainstem Colorado River in Glen and Grand Canyons supports very few species or individuals of native mayflies, stoneflies, or caddisflies because of a combination of stressors, including altered temperature regimes and a pronounced varial zone (Stevens, Shannon, et al. 1997; Kennedy et al. 2016). Some tributaries of the Colorado River, along with backwaters and off-channel ponds, have higher diversity and densities of mayflies, stoneflies, and caddisflies compared to the mainstem. (2018 Expanded Non-Native Aquatic Species Management Plan EA) |
| -- |
-- |
-- |
|---|
|
|