Midges (Chironomidae)
Midges are small, delicate flies, resembling mosquitoes but do not bite. Larvae are usually found in sediments, and can occur in everything ranging from highly polluted conditions to relatively clean water. [1] [2]
The chironomid fauna of the Colorado River in Grand Canyon is depauperate in comparison with other western rivers (e.g., Sublette and Sublette 1979, Wolz and Shiozawa 1995, Spindler 1996). Our collections include 38 species in 23 genera and 4 subfamilies. The fauna is dominated by the Orthoeladiinae (23 species), with 5 abundant species: Cricotopus annulator > Cricotopus globistylus > Eukiefferiella claripennis > Orthocladius rivicola > Tvetenia vitracies. The fauna includes 12 Chironominae species, with Chironomtts spp. regularly found in low densities in pool and backwater habitats floored with fine sand or silt, and with Procladius bellus, Paracladius conversus, Chironornus decorus, C. sp. 1, and C. sp. 2 collected only in the ULM segment. [3]
Midges detected or likely to occur in Grand Canyon [4]
TANYPODINAE
DIAMESlNAE
ORTHOCLADIINAE
- Cardiocladius platypus
- Cricotopus annulator
- Crirotopus blinni
- Crirotopus globistylus
- Crirotopus herrmanni
- Crirotopus infuscatus
- Cricotopus trifacsia
- Undet. Cricotopus sp.
- Eudactylocladius dubitatus
- Eukiefferiella claripennis
- Eukiefferiella coerulescens
- Eukiefferiella ilkeyensis
- Undet. Eukiefferiella sp.
- Undet. Limnophyes sp.
- Metriocnemus stevensi
- Orthocladius frigidus
- Orthocladius lutipes
- Orlhocladius mallochi
- Orthocladius rivicola
- Undet. Orthocladius sp.
- Paracladius conversus
- Parakiefferiella subaterrima
- Parametriocnemus lundbeckii
- Paraphaenocladius exagitans
- Pseudosmittia nanseni
- Undet. Pseudosmittia sp.
- Tvetenia vitracies
CHIRONOMlNAE
Chironomini
- Apedilum subcinctum
- Chironomus decorus
- Chironomus utahensis
- Chironomus sp. 1
- Chironomus sp. 2
- Cyphonoe1la gibbera
- Phaenospectra profusa
- Polypedilum apicatuim
- Polypedilum obelos
- Undet. Polypedilum sp.
Tanytarsini
- Cladotanytarsus marki
- Rheotanytarsus hamatus
- Undet. Micropsectra sp.
Blackflies (Simuliidae)
Blackflies are sometimes called a buffalo gnat, turkey gnat, or white socks. Most black flies gain nourishment by feeding on the blood of mammals, including humans, although the males feed mainly on nectar. They are usually small, black or gray, with short legs, and antennae. They are a common nuisance for humans, and many U.S. states have programs to suppress the black fly population. Eggs are laid in running water, and the larvae attach themselves to rocks. Breeding success is highly sensitive to water pollution.[2] The larvae use tiny hooks at the ends of their abdomens to hold on to the substrate, using silk holdfasts and threads to move or hold their place. They have foldable fans surrounding their mouths. The fans expand when feeding, catching passing debris (small organic particles, algae, and bacteria). The larva scrapes the fan's catch into its mouth every few seconds. Black flies depend on lotic habitats to bring food to them. They will pupate under water and then emerge in a bubble of air as flying adults. They are often preyed upon by trout during emergence. [5]
Mayflies (Ephemeroptera)
Ephemeroptera are aquatic insects that often go through many nymph stages (living in water) and two flying stages (the subimago and the imago). They are the only insects to have two flying stages, and can be recognized by their three caudal filaments (tails) at the tip of the abdomen, and a single claw on each leg. This differentiates them from the closely related stoneflies which have two tarsal claws. The flying stages are characterized by relatively large forewings, which are usually kept upright, and reduced or nonexistent hind wings.
The first stage of the life of a mayfly is the nymph (larva), which not only looks very different from the adult, but lives in the water. When the nymphs hatch from the eggs, they are less than 1 mm long. They have no gills at first, and their body shape varies according to habitat. For example, those that burrow (such as Ephemera) have more cylindrical bodies, whereas those that slide under rocks (such as Heptagenia) are flatter. Those in the genus Caenis crawl on mossy stones and vegetation, so they have short bodies with squat legs. Ephemeroptera nymphs may grow to anywhere from 4 mm to 3 cm long. They are generally camouflaged against their background. The number of molts a nymph goes through on its way to becoming an adult does not depend on its nutrition, but the increase in size that comes with each molt does.
In older nymphs, gills are found in pairs on each segment of the abdomen (see pictures below). The gills extend from the sides of the body and are oval-shaped. These gills beat to control the flow of water through the body, which also controls the amount of oxygen and salt that flows through the body. Nymphs in still waters generally have larger gills, and those in running water have smaller gills; this allows the nymphs of each habitat to get their optimum flow of water. Not only do the gills function in uptake of water, salt, and oxygen, but they also send water off at right angles to the body. This is used to mislead predators. If the water simply flowed out the back of the nymph's body, predators would know that the nymph was sitting at the beginning of the stream. However, since they send water away from their bodies at several points, the nymphs are not as easy to track.
When it comes time for the last nymph stage to molt into a subimago (the first flying stage), the guts empty out and the mid-gut section fills with air. Often, many nymphs will then simultaneously let go of their hold on their anchor in the water and float up to the top. Once they reach the air, the cuticle splits open on the thorax and the wings come out. This is the time of greatest vulnerability in their lives as they float on the water before they are strong enough to fly. The subimago has short hairs on the wings and on the body; the wings are dull and pigmented. Once it gains some strength, it flies from the water to some form of shelter such as a tree, long grass, or the underside of a bridge and molts again within 24 to 48 hours. Thisadditional molt allows the legs and tails of the insect to grow more. Longer tails give more stability in flight, and longer legs make it easier for the male to grasp the female in mating.
The imago (the final adult stage) has shiny, hairless wings. The longer legs and tails allow for more rapid flight. The corrugation of the wings protects them by making them more flexible and therefore less vulnerable to wind damage. The imago mates and dies within a few hours to a day. (Harker, 1989) This short adult life is what gives the order its name from the Greek ephemeros meaning "lasting but a day." [6]
Mayflies detected or likely to occur in Grand Canyon [7]
- Baetidae Acentrella insignificans (McDunnough 1926)
- Baetidae Baetis adonis (Traver 1935)
- Baetidae Baetis magnus (McCafferty and Waltz 1986)
- Baetidae Baetis notos (Allen and Murvosh 1987)
- Baetidae Baetis tricaudatus (Dodds 1923)
- Baetidae Baetodes arizonensis (Koss 1972)
- Baetidae Baetodes edmundsi (Koss 1972)
- Baetidae Camelobaetidius
- Baetidae Cloeodes excogitatus (Waltz and McCafferty 1987)
- Baetidae Fallceon quilleri (Dodds 1923)
- Ephemerellidae Ephemerella excrucians (Walsh 1862)
- Heptageniidae Epeorus longimanus (Eaton 1885)
- Leptohyphidae Leptohyphes zalope (Traver 1958)
- Leptohyphidae Tricorythodes explicatus (Eaton 1892)
- Leptophlebiidae Choroterpes inornata (Eaton 1892)
- Leptophlebiidae Paraleptophlebia memorialis (Eaton 1884)
- Leptophlebiidae Thraulodes speciosus (Traver 1934)
- Leptophlebiidae Traverella albertana (McDunnough 1931)
Stoneflies (Plecoptera)
The Plecoptera are an order of insects, commonly known as stoneflies. All species of Plecoptera are intolerant of water pollution, and their presence in a stream or still water is usually an indicator of good or excellent water quality.[8]
Stoneflies detected or likely to occur in Grand Canyon [9]
- Chloroperlidae Suwallia pallidula (Banks 1904)
- Chloroperlidae Sweltsa lamba (Needham and Claassen 1925)
- Nemouridae Malenka coloradensis (Banks 1897)
- Perlidae Hesperoperla pacifica (Banks 1900)
- Perlodidae Isoperla mormona (Banks 1920)
- Perlodidae Isoperla quinquepunctata (Banks 1902)
- Taeniopterygidae Taeniopteryx parvula (Banks 1918)
Caddisflies
Caddisflies resemble moths, but wings are hairy instead of scaly. Forewings are usually dark, sturdy, sometimes with striking color patterns, held tightly together roof-like over the abdomen when at rest. Hindwings often clear, relatively delicate, and hidden under forewings when at rest. Antennae usually very long, threadlike, with many segments. The aquatic larvae have three pairs of legs and a soft, elongate, segmented abdomen usually hidden inside a case. Most species live in a mobile case constructed from plant material, algae, grains of sand, pieces of snail shells, or entirely of silk. The case is held together with strands of silk secreted by the larva. In some species the case is attached to a rock, log, or other underwater surface; a few species have no case and are free-living. The case's particular shape and construction material is distinctive of the family and/or genus, and can be used in identification. For example: Helicopyschidae larvae use sand grains to build spiral cases that resemble small snail shells. [10]
Caddisflies detected or likely to occur in Grand Canyon [11]
- Brachycentridae Brachycentrus occidentalis (Banks 1911)
- Brachycentridae Micrasema bactro (Ross 1938)
- Glossosomatidae Protoptila erotica (Ross 1938)
- Helichopsychidae Helichopsyche mexicana (Banks 1901)
- Hydropsychidae Ceratopsyche cockerelli (Banks 1905)
- Hydropsychidae Ceratopsyche venada (Ross 1941)
- Hydropsychidae Cheumatopsyche pinula (Denning 1952)
- Hydropsychidae Hydropsyche auricolor (Ulmer 1905)
- Hydroptilidae Alisotrichia arizonica (Blickle and Denning 1977)
- Hydroptilidae Hydroptila ajax (Ross 1938)
- Hydroptilidae Hydroptila argosa (Ross 1938)
- Hydroptilidae Hydroptila pecos (Ross 1941)
- Hydroptilidae Ithytrichia mexicana (Harris and Contreras-Ramos 1989)
- Hydroptilidae Mayatrichia ayama (Mosely 1937)
- Hydroptilidae Ochrotrichia argentea (Flint and Blickle 1972)
- Hydroptilidae Ochrotrichia dactylophora (Flint 1965)
- Hydroptilidae Ochrotrichia ildria (Denning and Blickle 1972)
- Hydroptilidae Ochrotrichia logana (Ross 1941)
- Hydroptilidae Ochrotrichia rothi (Denning and Blickle 1972)
- Hydroptilidae Ochrotrichia tarsalis (Hagen 1861)
- Lepidostomatidae Lepidostoma ormea (Ross 1946)
- Leptoceridae Ceraclea annulicornis (Stephens 1836)
- Leptoceridae Nectopsyche dorsalis (Banks 1901)
- Leptoceridae Nectopsyche lahontensis (Haddock 1977)
- Leptoceridae Oecetis arizonica (Denning 1951)
- Leptoceridae Oecetis avara (Banks 1895)
- Leptoceridae Oecetis disjuncta (Banks 1920)
- Limnephilidae Hesperophylax d. designatus (Walker 1852)
- Limnephilidae Limnephilus apache (Flint 1965)
- Limnephilidae Limnephilus spinatus (Banks 1914)
- Limnephilidae Psychoglypha schuhi (Denning 1970)
- Limnephilidae Psychoglypha subborealis (Banks 1924)
- Philopotamidae Chimarra primula (Denning 1950)
- Philopotamidae Chimarra ridleyi (Denning 1941)
- Philopotamidae Dolophilodes novusamericana (Ling 1938)
- Polycentropodidae Polycentropus gertschi (Denning 1950)
- Psychomyiidae Psychomyia flavida (Hagen 1861)
- Rhyacophilidae Rhyacophila brunnea (Banks 1911)
- Rhyacophilidae Rhyacophila jenniferae (Peck 1978)
- Rhyacophilidae Rhyacophila pellisa (Ross 1938)
- Rhyacophilidae Rhyacophila rotunda (Banks 1924)
- Sericostomatidae Gumaga griseola (McLachlan 1871)
- Uenoidae Neothremma alicia (Dodds and Hisaw 1925)
- Uenoidae Oligophlebodes sierra (Ross 1944)
Freshwater amphipods, scuds
Gammarus lacustris
 Gammarus lacustris: an introduced non-native freshwater shrimp (amphipod) G. lacustris is semi-transparent and lacks a webbed tail. It may be colorless, brown, reddish or bluish in color, depending on the local environment. It has seven abdominal segments, a fused cephalothorax, and two pairs of antennae. Unlike other crustaceans, amphipods lack carapaces and have laterally compressed bodies. The female carries eggs in a brood pouch on its ventral side. G. lacustris in higher elevations were more likely to have fewer but larger eggs than those living at lower elevations. G. lacustris undergoes several molts and juveniles resemble the adult. G. lacustris plays an important role in many of the freshwater ecosystems that it inhabits. It is a detritivore and may also consume algae, mainly diatoms. It is considered an indicator species for the overall health and stability of the ecosystem. As a small aquatic invertebrate G. lacustris is an important food source for many organisms. [12]
Clams and Mussels
Fingernail clams (Sphaeriidae)
Fingernail clams are small "mostly about the size of a finger or thumbnail" bottom-dwelling, filter-feeders found in ponds, lakes and streams. They are native and can be quite abundant, providing food for a variety of animals and producing large accumulations of empty shells. These shells can be quite fragile compared to introduced Asian clams of the family Corbiculidae. [13]
- Triangular peaclam (Pisidium variable) [14]
- Walker peaclam (Pisidium walkeri) [15]
Quagga mussel (Dreissena rostriformis bugensis)
A highly invasive mussel prone to causing biofowling of water-related infrastructure.
Snails
Physella
Physella is a genus of small, left-handed, air-breathing freshwater snails.
Fossaria obrussa
Fossaria obrussa is a species of air-breathing freshwater pond snails.
New Zealand mudsnails (Potamopyrgus antipodarum)
The New Zealand mudsnail is a tiny aquatic snail that inhabits lakes,
rivers, streams, reservoirs and estuaries. In addition to mud, the snail can also be found lurking
on rock or gravel surfaces, aquatic vegetation, or woody debris. New Zealand mudsnail are
highly adaptable to diverse climates and can tolerate a broad range of aquatic conditions such
as temperature, salinity, turbidity, water velocity, and stream productivity. In the United
States, New Zealand mudsnail populations are comprised almost entirely of self‐cloning
parthenogenetic females. The brood size of an individual female
ranges from 20‐120 embryos, each of which may mature to produce an average of 230
offspring per year. A single female mudsnail can result in a colony of 40 million snails in one year. Some fish and birds feed on New Zealand mudsnail, but
the rigid operculum and thick shell wall enable many snail to pass
through the digestive system of predators unharmed. [16] New Zealand mudsnails were introduced below Glen Canyon Dam around 1995.
Worms
Lumbricidae
The Lumbricidae are a family of earthworms which includes most of the well-known earthworm species.
Lumbriculidae
The Lumbriculidae are a family of microdrile oligochaetes common in freshwater environments, including streams, lakes, marshes, wells and groundwater. They should not be confused with the earthworm family Lumbricidae. Many species and genera are highly endemic.
Naididae
The Naididae (formerly known as Tubificidae) are a family of clitellate oligochaete worms like the sludge worm, Tubifex tubifex. They are key components of the benthic communities of many freshwater and marine ecosystems. In freshwater aquaria they may be referred to as detritus worms.
Planaria
Dugesia
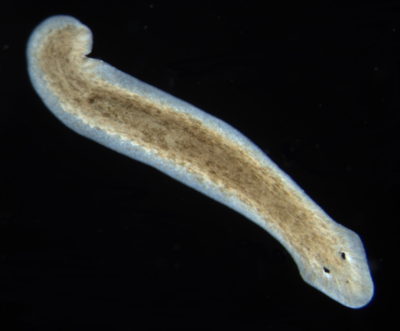 Flatworm (Planaria, Dugesia) Dugesia (pronounced, d(y)üˈjēzh(ē)ə) is a genus of dugesiid triclads that contains some common representatives of the class Turbellaria. These common flatworms are found in freshwater habitats of Africa, Europe, Middle East, Asia, and Australia.
|





